Alpha-synuclein
Parkinson’s disease (PD) is one of the debilitating neurodegenerative diseases associated to the formation of amyloid fibrils. In fact, PD is tightly associated with the assembly of proteins into highly ordered high-molecular weight species and the propagation of protein aggregates between neurons in the central nervous system. The protein involved is α-synuclein (140 AA).
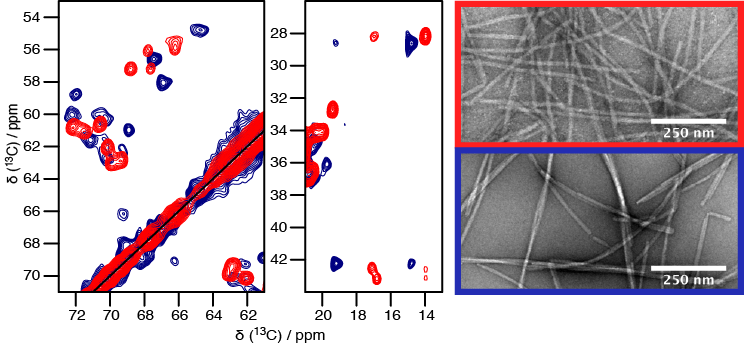
Solid-state NMR has enabled considerable progress in the structural determination of α-synuclein fibrils or fragments thereof. However, a comparison of the published spectra suggests the presence of different polymorphs. Our collaboration partners are now able to prepare homogeneous samples, i.e. several polymorphs as different samples, and we obtained the solid-state NMR sequential assignments of two fibrillar forms of α-synuclein. With the assignments in hand we were able to compare the structure of these two different polymorphs of α-synuclein, which also display contrasting biochemical properties, toxicity, and tropism for cells (see figure).
Collaboration with external pageRonald Melkicall_made (Paris-Saclay Institute of Neuroscience, France) and external pageAnja Böckmanncall_made (IBCP, Lyon France)