Interactions between small molecules and amyloids
The characterization of amyloid pharmacophores by probing with small molecules of interest, e. g. dyes, markers and drugs, is of crucial importance not only for rational design of drugs and markers, but also for therapeutic intervention.
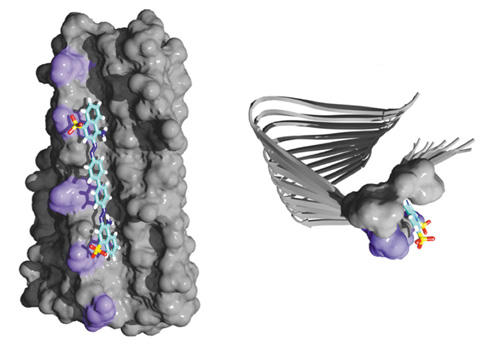
With the atomic-resolution structure of amyloid fibrils formed by HET-s(218-289) in hand, we have been able to develop the methodology to investigate the water-accessibility of amyloids on a residue-by-residue basis and to determine the details of the interaction of amyloids with small molecules, more specifically the dye Congo red.
Although traditionally amyloid fibrils are characterized by their binding to Congo red and thioflavin T, the specificity, sensitivity and binding properties of these small molecular probes to amyloid fibrils were poorly understood and controversial. Our NMR results have led to an atomic-resolution 3D structure of Congo red in complex with HET-s. Interestingly, these data also showed that point-mutated HET-s, which shares the structure with wild-type HET-s, is non-congophilic, thus questioning Congo red as the “gold standard” for the diagnosis of amyloids.
Collaboration with Adriano Aguzzi (University Hospital of Zurich) and external pageAnja Böckmanncall_made (IBCP Lyon, France).
